The Art and Alchemy of Saggar Fired Pottery: Exploring Ferric Chloride’s Transformative Role in Modern Ceramics
Table of Contents
What you will learn
📋 What You'll Learn
This guide walks you through the art and alchemy of saggar fired pottery: exploring ferric chloride’s transformative role in modern ceramics with detailed instructions.
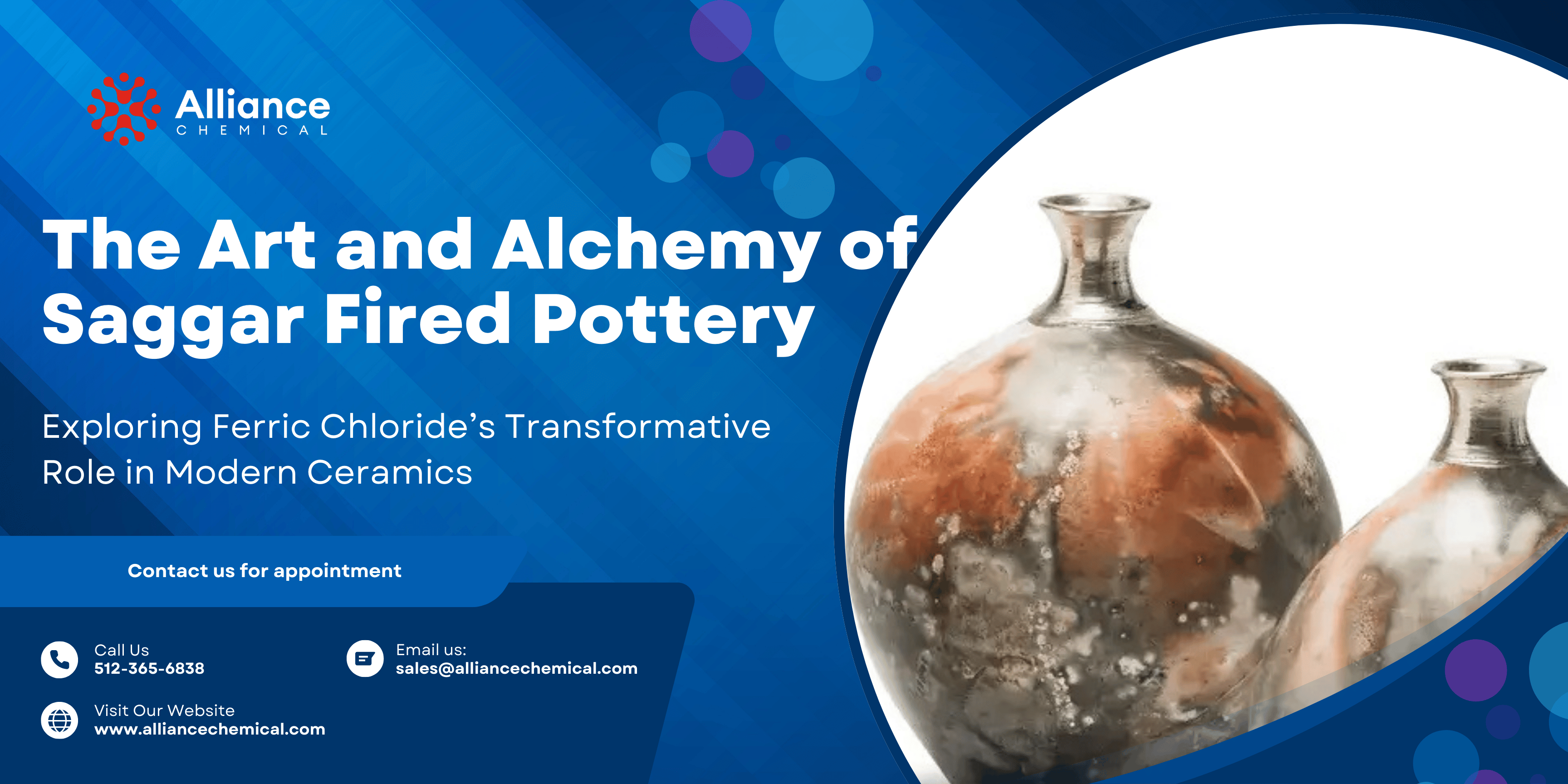
In the world of ceramics, there exists a realm where chemistry and chance collide, where ancient techniques are reborn to create breathtakingly modern art. This is the realm of saggar firing. By introducing a powerful chemical actor like Ferric Chloride into this controlled, atmospheric process, potters can create surfaces of unparalleled depth, color, and unpredictability. This definitive guide explores the history and science of saggar firing, provides a detailed walkthrough of the process, and delves into the artistic possibilities that await when you harness the transformative power of Ferric Chloride.
The Art & Science of Atmospheric Firing
At its core, saggar firing is a method of creating a localized, miniature atmosphere around a ceramic piece within the larger kiln. The term "saggar" comes from "safeguard," as these containers were historically used to protect delicate porcelain from the harsh wood or coal ash of the kiln.
Modern alternative potters, however, have inverted this purpose. Instead of keeping things out, the saggar is used to trap things in. By placing combustible organic materials and volatile chemicals inside a sealed container with the pottery, the artist creates a unique, oxygen-starved micro-environment during the firing. As these materials heat up, they release gases, fumes, and smoke that react with the clay surface, producing a rich tapestry of colors and patterns that can never be precisely duplicated.
Ferric Chloride: The Master Colorant
While many chemicals can be used in saggar firing, Ferric Chloride (FeCl₃) is a favorite for the stunning range of warm, earthy tones it produces.
The Chemistry of Fuming
Ferric Chloride is a volatile iron salt. As the kiln heats up, the Ferric Chloride solution vaporizes, creating a dense fume rich in iron ions. These hot, gaseous ions are highly reactive and desperately seek to bond with other elements. They are drawn to the silica and alumina in the clay body of the pot, embedding themselves in the surface and creating the signature warm palette of saggar firing.
- In an oxygen-rich (oxidation) atmosphere, the iron will produce bright reds, oranges, and yellows.
- In an oxygen-starved (reduction) atmosphere, the colors can shift to deeper browns, blacks, and even subtle metallic sheens.
This dynamic interplay between the chemical fumes and the kiln atmosphere is what makes every saggar-fired piece a unique record of its journey through fire.
A Step-by-Step Guide to the Saggar Firing Process
This process is a beautiful dance between preparation and serendipity. While you can't control the final outcome, careful preparation is key to setting the stage for amazing results.
Step 1: Preparing the Vessel (The Canvas)
The surface of your pot is your canvas. A smooth, dense surface will capture the fumed patterns most vividly.
- Choose a Clay Body: A fine, light-colored stoneware or porcelain clay body is ideal as it provides a neutral background for the colors.
- Burnish, Burnish, Burnish: The single most important surface preparation is burnishing. After the pot is bone-dry, rub the surface meticulously with the back of a spoon or a smooth stone. This aligns the clay particles, creating a tight, smooth, and slightly glossy surface that will not be glazed.
- Bisque Fire: Fire the burnished pot to a low bisque temperature (around Cone 08 or 955°C / 1751°F). This makes the pot durable enough to handle but keeps it porous enough to accept the fuming.
Step 2: Preparing the Saggar (The Micro-Kiln)
You can use a traditional ceramic saggar or create a disposable one. For beginners, a simple foil saggar is an excellent and affordable option.
How-To: Create a Foil Saggar
- Place your bisque-fired pot on a large sheet of heavy-duty aluminum foil.
- Sprinkle a bed of fine sawdust or wood shavings around the base of the pot.
- Carefully arrange your combustible materials (see next step) and chemical-soaked materials around the pot, ensuring they make contact with the surface in some places.
- Loosely wrap the foil up and around the pot, creating a sealed but not airtight package. You want the gases to be trapped, but have a small escape route to prevent pressure buildup.
Step 3: Loading the Organics and Chemicals
This is where the true artistry begins. The materials you choose will define the final pattern.
- Combustibles: Sawdust, wood shavings, and straw create a reduction atmosphere as they smolder.
- Organics for Pattern: Wrap pieces in seaweed, banana peels, or strands of copper wire. Sprinkle coffee grounds or salt crystals onto the surface.
- Applying the Ferric Chloride: Wearing full PPE, you can apply the 40% Ferric Chloride solution by brushing it directly onto the pot, or by soaking materials like string or steel wool in it and wrapping them around the piece.
Step 4: The Firing
Saggar firing is a low-temperature firing process. The goal is not to mature the clay body, but to activate the chemicals and combustibles.
| Segment | Rate | Temperature | Hold Time |
|---|---|---|---|
| 1 | 150°C (302°F) per hour | 600°C (1112°F) | 30 minutes |
| 2 | 100°C (212°F) per hour | 850°C (1562°F) | 15 minutes |
After the hold, turn the kiln off and allow it to cool completely and naturally. Do not open the kiln until it is cool to the touch. This slow cooling is part of the process and prevents thermal shock.
Advanced Techniques: Expanding Your Palette
Once you've mastered the basics with Ferric Chloride, you can introduce other metal salts to create a wider range of colors.
- Copper Carbonate: Produces a range of colors from subtle pinks and reds in reduction to vibrant greens in oxidation.
- Cobalt Carbonate: Yields beautiful shades of blue. Use very sparingly as it is extremely powerful.
- Steel Wool: When wrapped around a pot, plain steel wool will create rich, dark iron markings.
MANDATORY Safety Protocols for Saggar Firing
This process involves hazardous, corrosive chemicals and high temperatures. Safety is not optional.
- Chemical Handling: Ferric Chloride is corrosive and will stain anything it touches. Always wear chemical-resistant gloves, splash-proof safety goggles, and a protective apron when handling it. Work in a very well-ventilated area.
- Kiln Safety: Ensure your kiln is in good working order and is properly ventilated to the outside. The fumes produced during this firing are hazardous and should not be inhaled.
- Material Storage: Store all chemicals in their original, clearly labeled containers, separate from your main studio area and out of reach of children and pets.
- Disposal: Dispose of all spent saggars, ash, and chemical residues according to your local hazardous waste regulations.







