How to Make 70% Isopropyl Alcohol from 99% Isopropyl Alcohol
Welcome to the wonders of dilution! In the potion class today, we’ll turn that potent 99% isopropyl alcohol into a kinder, gentler, yet equally clean 70% solution. Whether you’re a budding scientist, a clean freak, or just someone who’s really into their percentages, you’ve come to the right place. Today, we’re going to transform that super potent 99% isopropyl alcohol into the more versatile and widely-used 70% solution. Grab your lab coats (or aprons), and let’s embark on a journey from high-purity to high-practicality. Get ready to mix things up!
Table of Contents
- Introduction
- Understanding Isopropyl Alcohol
- Why Dilute to 70%?
- Materials and Safety Equipment
- The Dilution Recipe
- The Science of Mixing
- Safety Precautions
- Uses for 70% Isopropyl Alcohol
- Storing Your 70% Solution
- FAQs
- Conclusion
Understanding Isopropyl Alcohol
What is Isopropyl Alcohol?
Isopropyl alcohol, also known as isopropanol or IPA, is a colorless, flammable chemical compound with a strong odor. It’s a versatile substance used in various industries due to its excellent solvent properties. It’s commonly found in antiseptics, disinfectants, and cleaning materials. Its ability to evaporate quickly and leave nearly zero oil traces makes it ideal for cleaning electronic devices, glass, and metals.
The Difference Between 70% and 99%
The concentration of isopropyl alcohol in a solution determines its effectiveness and usage. A 70% concentration is often preferred for disinfection because it penetrates cell walls more completely, thereby killing microbes more effectively. In contrast, 99% isopropyl alcohol, due to its extremely high concentration, evaporates too quickly to effectively kill bacteria and viruses. This section will delve into why a 70% concentration is more suitable for most disinfecting purposes.
The Difference Between 70% and 99% Isopropyl Alcohol
Understanding the difference between 70% and 99% isopropyl alcohol is crucial for its effective use. The key difference lies in their respective concentrations and how they interact with bacterial cells and viruses.
99% Isopropyl Alcohol: This concentration is more potent due to its high purity level. It’s primarily used in industrial settings and for specialized cleaning where rapid evaporation and absence of water are required. However, for disinfection purposes, its high evaporation rate is a disadvantage. It evaporates so quickly that it doesn’t stay on surfaces long enough to effectively kill microbes.
70% Isopropyl Alcohol: A 70% solution is more effective for general disinfection. This concentration strikes a perfect balance, allowing the alcohol to penetrate the cell walls of bacteria and viruses effectively. The presence of water in the solution slows down the evaporation rate, giving the alcohol enough time to act on the microorganisms. This makes 70% isopropyl alcohol the preferred choice for use in medical environments, laboratories, and for household cleaning and disinfection.
It’s important to understand that while 99% isopropyl alcohol can be more effective in certain industrial applications, for disinfecting purposes, the 70% solution is often more suitable. In the following sections, we will guide you through diluting 99% isopropyl alcohol to achieve the more versatile 70% concentration.
-
 Isopropyl Alcohol 99.9% ACS Reagent Grade$22.00 – $3,600.00
Isopropyl Alcohol 99.9% ACS Reagent Grade$22.00 – $3,600.00 -
 Isopropyl Alcohol 70% USP Grade$25.00 – $3,700.00
Isopropyl Alcohol 70% USP Grade$25.00 – $3,700.00 -
 Isopropyl Alcohol 99%$19.00 – $3,500.00
Isopropyl Alcohol 99%$19.00 – $3,500.00
Why Dilute to 70%?
Diluting 99% isopropyl alcohol to a 70% concentration is not just a matter of preference but a strategic decision based on scientific principles. The effectiveness of isopropyl alcohol as a disinfectant is significantly influenced by its concentration. Here’s why a 70% solution is often more effective than higher concentrations:
- Improved Microbial Killing Power: A 70% concentration of isopropyl alcohol is more effective at killing microbial cells. This is because the presence of water in the solution facilitates a slower evaporation process, allowing the alcohol more time to penetrate the cell walls of bacteria and viruses, leading to their destruction.
- Safety and Reduced Toxicity: Lower concentrations of alcohol are generally safer to use. They pose a lower risk of causing skin irritation and are less toxic in case of accidental ingestion. This makes 70% isopropyl alcohol a safer choice for household and medical settings.
- Better for Cleaning Surfaces: The slower evaporation rate of a 70% solution makes it more suitable for cleaning surfaces. It stays on surfaces longer than a 99% solution, ensuring thorough cleaning and disinfection.
- Cost-Effectiveness: Diluting 99% isopropyl alcohol to make a 70% solution can be more cost-effective. You can achieve more volume of disinfectant from a highly concentrated solution, making it an economical choice for large scale or frequent use.
In the following sections, we will explore the materials needed and provide a detailed guide on how to safely dilute 99% isopropyl alcohol to achieve a 70% concentration for optimal use.
Materials and Safety Equipment
Before you begin the process of diluting 99% isopropyl alcohol to 70%, it’s important to gather the necessary materials and safety equipment. This will ensure that the process is not only effective but also safe. Here’s what you’ll need:
Materials Required
- 99% Isopropyl Alcohol: This will be the primary ingredient that you’ll be diluting.
- Distilled Water: It’s crucial to use distilled water to maintain the purity of the solution. Tap water can introduce contaminants or minerals that can interfere with the efficacy of the alcohol.
- Measuring Tools: Accurate measurement is key. Use measuring cups or a digital scale for precision.
- Storage Container: A clean, airtight container for storing your 70% isopropyl alcohol solution. Glass or plastic containers that have been thoroughly cleaned and dried are suitable.
Safety Equipment
- Gloves: Wear protective gloves to avoid skin irritation and to protect your hands from spills.
- Safety Goggles: Protect your eyes from potential splashes by wearing safety goggles.
- Well-Ventilated Area: Always work in a well-ventilated area to avoid inhaling fumes, which can be harmful.
- Fire Safety: Remember that isopropyl alcohol is flammable. Keep it away from heat sources, sparks, and open flames.
Having these materials and safety equipment ready will make the dilution process smoother and safer. Next, we’ll dive into how to calculate the correct proportions for dilution and provide a step-by-step guide to achieving the desired 70% concentration.

The Dilution Recipe
Ready with your materials and safety gear? Great! Let’s make diluting 99% isopropyl alcohol to 70% concentration as simple as possible. Follow these straightforward steps:
Understanding the Basics:
We’ll use a basic formula to mix the solution. The formula is: Concentrationbefore × Volumebefore = Concentrationafter × Volumeafter. Here’s what each term means:
- Concentration tells us how strong the alcohol is.
- Volume is how much alcohol we have.
- ‘Before’ is what we start with, and ‘after’ is what we want to end up with.
Your Starting Point:
You have 100ml of really strong alcohol – 99% concentration.
Your Goal:
You want to make this alcohol less strong, specifically to a 70% concentration.
Using the Formula:
Let’s put our numbers into the formula:
- Your starting concentration (before) is 99%.
- Your starting volume (before) is 100ml.
- Your desired concentration (after) is 70%.
Calculating the New Volume:
We rearrange our formula to find the new volume (Volumeafter). It looks like this: Volumeafter = (Concentrationbefore × Volumebefore) / Concentrationafter. When we plug in our numbers, we find that the new volume should be 141ml.
How Much Water to Add:
Since we’re starting with 100ml, and we need 141ml in total, we just need to add enough water to make up the difference. That’s 41ml of water.
In a Nutshell:
So, there you have it: Mix 100ml of 99% alcohol with 41ml of water, and voilà, you’ve got 141ml of 70% alcohol solution. Simple!
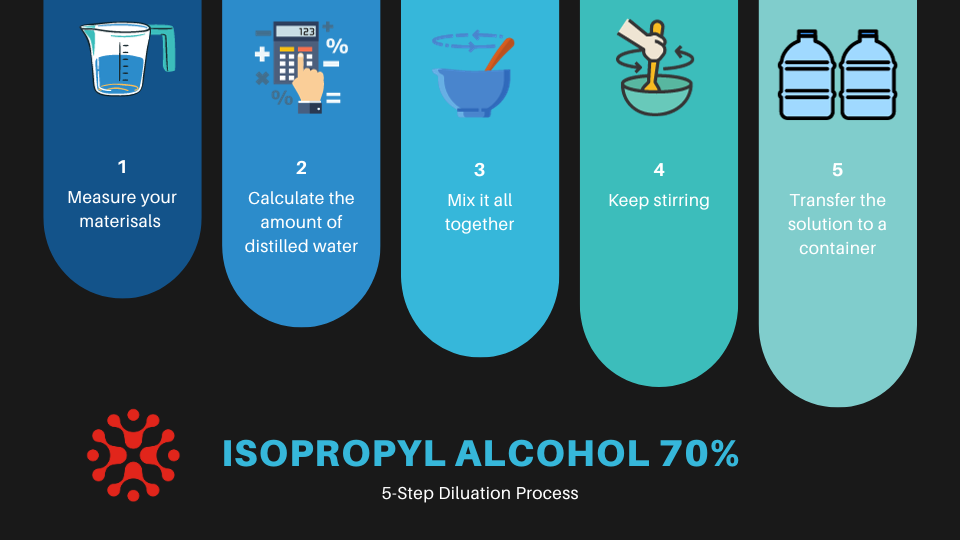
Step-by-Step Dilution Guide
This guide will walk you through the process of diluting 99% isopropyl alcohol to a 70% concentration, step by step. By following these instructions, you can ensure accuracy and safety.
- Preparation: Begin by ensuring your workspace is clean, well-ventilated, and away from any heat sources. Have all your materials and safety equipment at hand.
- Measuring Alcohol: Use a measuring cup or digital scale to measure the exact amount of 99% isopropyl alcohol you’ll be diluting. For example, if you’re making 200ml of 70% solution, measure out 200ml of 99% alcohol.
- Calculating Water Volume: Refer to the dilution formula provided in the previous section to calculate the amount of distilled water needed. Subtract the volume of alcohol from the total desired volume of the 70% solution to get the water volume.
- Mixing: Slowly add the calculated amount of distilled water to the alcohol. Mix the solution by gently stirring or shaking, ensuring a thorough blend.
- Finalizing the Solution: Once fully mixed, transfer the solution to a clean, airtight container. Label the container with the concentration and the date of preparation for future reference.
- Testing: If possible, test the final concentration using appropriate equipment to ensure accuracy. This step is especially important in professional settings.
Following these steps will give you a 70% isopropyl alcohol solution, ideal for disinfection and cleaning purposes. Next, we will explore the science behind the mixing process, explaining why this method is effective.
The Science of Mixing
The process of diluting isopropyl alcohol from 99% to 70% is not just a physical mixture but a demonstration of the principles of chemistry. When water is added to isopropyl alcohol, it affects the alcohol’s volatility (or rate of evaporation) and its ability to penetrate and destroy microbial cell walls. The presence of water slows down the evaporation rate of alcohol, allowing it to remain on surfaces longer, which is crucial for effective disinfection.
Safety Precautions
While diluting and using isopropyl alcohol, safety should always be a priority. Always work in a well-ventilated area and wear protective gloves and safety goggles. Keep the alcohol away from heat sources, sparks, and open flames, as it is highly flammable. Avoid skin contact and inhalation of fumes as much as possible, and always store the alcohol in a cool, dry place, away from children and pets.
Uses for 70% Isopropyl Alcohol
70% isopropyl alcohol is widely used due to its effective disinfecting properties. It’s commonly used in medical settings for sanitizing hands and cleaning equipment. In households, it’s excellent for cleaning electronics, glass, and metal surfaces, as it doesn’t leave streaks and evaporates quickly. It’s also used in beauty and skincare products, such as toners and astringents, due to its antiseptic properties. However, it’s important to use it with caution on sensitive skin.
Storing Your 70% Solution
Proper storage of your 70% isopropyl alcohol solution is crucial for maintaining its efficacy and longevity. Store the solution in a cool, dry place away from direct sunlight and heat sources. Use an airtight container to prevent evaporation and contamination. It’s also advisable to label the container with the concentration and the date of preparation for easy identification and to keep track of its shelf life.
FAQs
- Can I use tap water for dilution?
- No, it’s best to use distilled water to avoid introducing impurities and minerals that can affect the solution’s effectiveness.
- How long can I store the 70% alcohol solution?
- When stored properly, a 70% isopropyl alcohol solution can last for several years. However, always check for any signs of contamination or evaporation before use.
- Is 70% alcohol solution safe for all surfaces?
- While it’s safe for most surfaces, avoid using it on sensitive materials like certain plastics and fabrics, as it may cause damage or discoloration.
- Can I increase the alcohol concentration for better disinfection?
- No, higher concentrations evaporate too quickly and are less effective for disinfection. Stick to the 70% solution for optimal results.
Conclusion
In conclusion, creating a 70% isopropyl alcohol solution from a 99% concentration is a straightforward process that offers a more effective and safer option for disinfection and cleaning. By understanding the science behind the dilution and adhering to safety precautions, you can prepare a versatile and cost-effective disinfectant for various uses. Remember to store your solution properly and always prioritize safety when handling chemical substances.